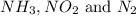Chemistry, 11.03.2020 05:11, kylaprather06
Consider three 1L flask at STP. Flask A contains NH3, flask B contains NO2 gas and flask C contain N2 gas. Which constains the largest number of molecules?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, princessroyal
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1


Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
Do you know the correct answer?
Consider three 1L flask at STP. Flask A contains NH3, flask B contains NO2 gas and flask C contain N...
Questions in other subjects:


Mathematics, 04.09.2020 08:01

English, 04.09.2020 08:01


History, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01



Mathematics, 04.09.2020 08:01