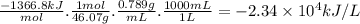Chemistry, 15.11.2019 17:31, jessicachichelnitsky
Ethanol (c2h5oh) is currently blended with gasoline as an automobile fuel. calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. ethanol has a density of 0.789 g/ml.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
Do you know the correct answer?
Ethanol (c2h5oh) is currently blended with gasoline as an automobile fuel. calculate the heat produc...
Questions in other subjects:


Geography, 15.06.2021 21:20

Mathematics, 15.06.2021 21:20


Computers and Technology, 15.06.2021 21:20

Advanced Placement (AP), 15.06.2021 21:20


Biology, 15.06.2021 21:20

Mathematics, 15.06.2021 21:20