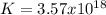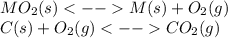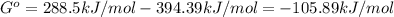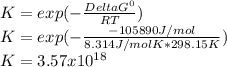Chemistry, 10.11.2019 07:31, lovelyheart5337
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g) delta g = 288.5 kj/mol
1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s)
2.) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 3
Similar questions


Chemistry, 25.07.2019 14:30, michaelortega5808
Answers: 1


Chemistry, 10.11.2019 04:31, shadowsnake
Answers: 1
Do you know the correct answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions in other subjects:


Mathematics, 11.10.2020 01:01



Mathematics, 11.10.2020 01:01


Mathematics, 11.10.2020 01:01