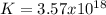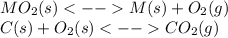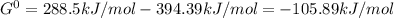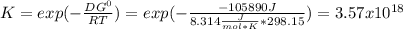Chemistry, 10.11.2019 04:31, shadowsnake
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g)delta g = 291.0 kj/mol1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s) .) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions in other subjects:

Mathematics, 19.10.2019 22:50

Mathematics, 19.10.2019 22:50

Mathematics, 19.10.2019 22:50



Social Studies, 19.10.2019 22:50


History, 19.10.2019 22:50


Mathematics, 19.10.2019 22:50