
Chemistry, 01.11.2019 06:31, brianortega1208
Aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin (c9h8o4) and acetic acid (c2h4o2). the balanced equation is: c4h6o3+c7h6o3→c9h8o4+c2h4o2.in a laboratory synthesis, a student begins with 5.00 ml of acetic anhydride (density = 1.08 g / ml) and 2.08 g of salicylic acid. once the reaction is complete, the student collects 2.07 g of aspirin. determine the limiting reactant for the reaction?
determine the theoretical yield of aspirin for the reaction?
determine the percent yield of aspirin for the reaction.

Answers: 3
Similar questions

Chemistry, 26.07.2019 15:00, jerry22591
Answers: 1

Chemistry, 12.08.2019 17:10, silveryflight
Answers: 1

Chemistry, 10.10.2019 23:30, makennahudson94
Answers: 1

Chemistry, 26.10.2019 00:43, gizmo50245
Answers: 3
Do you know the correct answer?
Aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h...
Questions in other subjects:

Physics, 25.04.2021 14:00

Biology, 25.04.2021 14:00




History, 25.04.2021 14:00

English, 25.04.2021 14:00

Geography, 25.04.2021 14:00

English, 25.04.2021 14:00


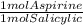 = 0.01507 mol aspirin * 180 g/mol = 2.71 g aspirin
= 0.01507 mol aspirin * 180 g/mol = 2.71 g aspirin



