Chemistry, 12.08.2019 17:10, silveryflight
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3). the balanced equation isc7h6o3 + c4h6o3 → c9h8o4 + hc2h3o2(a) what mass of acetic anhydride is needed to completely consume 1.00 x 10^2 g salicylic acid? (b) what is the maximum mass of aspirin (the theoretical yield) that could be produced in this reaction?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Do you know the correct answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3)....
Questions in other subjects:


Mathematics, 28.01.2020 18:42


Mathematics, 28.01.2020 18:42

Spanish, 28.01.2020 18:42

History, 28.01.2020 18:42





 .....(1)
.....(1)


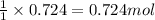 of acetic anhydride.
of acetic anhydride.