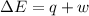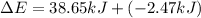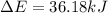Chemistry, 23.10.2019 00:30, kandigirl9990
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−3(aq)→caco3(s) co2(g) h2o(l) if 1 mol of caco3 forms at 298 k under 1 atm pressure, the reaction performs 2.47 kj of p−v work, pushing back the atmosphere as the gaseous co2 forms. at the same time, 38.65 kj of heat is absorbed from the environment. what is the value of δe for this reaction

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 05:00, skylarjohnson2683
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
Do you know the correct answer?
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−...
Questions in other subjects:




History, 13.01.2020 07:31

Mathematics, 13.01.2020 07:31


English, 13.01.2020 07:31




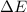 for this reaction is 36.18 kJ
for this reaction is 36.18 kJ