Which one of the following statements is true?
a. the enthalpy change for a reaction is inde...

Chemistry, 17.10.2019 19:00, unknown235
Which one of the following statements is true?
a. the enthalpy change for a reaction is independent of the state of the reactants and products.
b. enthalpy is an intensive property.
c. enthalpy is a state function.
d. h is the value of q measured under conditions of constant volume.
e. the enthalpy change of a reaction is the reciprocal of the δh of the reverse reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2
Do you know the correct answer?
Questions in other subjects:



History, 19.09.2019 01:00

Business, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00




Mathematics, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00


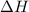 and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.
and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.



