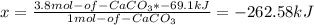Chemistry, 10.10.2019 22:10, BaileyElizabethRay
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is ca(oh)2(s) + co2(g) → caco3(s) + h2o(g) δh = –69.1 kj what is the enthalpy change if 3.8 mol of calcium carbonate is formed?

Answers: 3
Similar questions

Chemistry, 08.07.2019 10:30, emilyswinge4421
Answers: 1

Chemistry, 02.08.2019 17:20, Leonorareed5145
Answers: 3

Chemistry, 16.10.2019 17:10, Crxymia
Answers: 1

Chemistry, 23.10.2019 18:00, noahdavis4650
Answers: 1
Do you know the correct answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions in other subjects:


History, 04.11.2019 14:31





English, 04.11.2019 14:31

Mathematics, 04.11.2019 14:31

Biology, 04.11.2019 14:31



 are formed.
are formed.