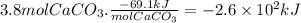Chemistry, 23.10.2019 18:00, noahdavis4650
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is
ca(oh)2(s) + co2(g) --> caco3(s) + h2o(g) ; δh = -69.1 kj
what is the enthalpy change if 3.8 mol of calcium carbonate is formed?
(a) -18 kj
(b) -69 kj
(c) -73 kj
(d) -260 kj

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
Do you know the correct answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions in other subjects:




Mathematics, 08.12.2020 06:30


Mathematics, 08.12.2020 06:30

Advanced Placement (AP), 08.12.2020 06:30

Mathematics, 08.12.2020 06:30

Physics, 08.12.2020 06:30