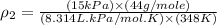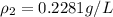Chemistry, 24.09.2019 19:00, saskiat1155
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine the new density and volume of the carbon dioxide if the temperature and pressure change to 75 oc and 15 kpa.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
Do you know the correct answer?
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine th...
Questions in other subjects:


Business, 15.06.2021 03:50

Mathematics, 15.06.2021 03:50


Mathematics, 15.06.2021 03:50


Mathematics, 15.06.2021 03:50

Chemistry, 15.06.2021 03:50



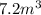 respectively.
respectively.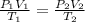
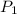 = initial pressure of gas = 10 kPa
= initial pressure of gas = 10 kPa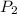 = final pressure of gas = 15 kPa
= final pressure of gas = 15 kPa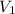 = initial volume of gas =
= initial volume of gas = 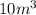
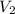 = final volume of gas = ?
= final volume of gas = ?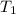 = initial temperature of gas =
= initial temperature of gas = 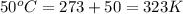
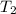 = final temperature of gas =
= final temperature of gas = 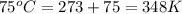
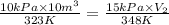
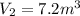
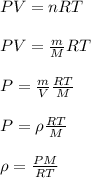
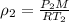
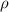 = new density
= new density