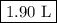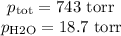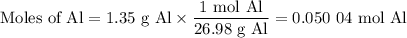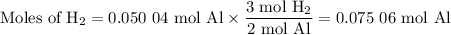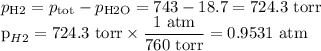Chemistry, 19.09.2019 03:00, davelopez979
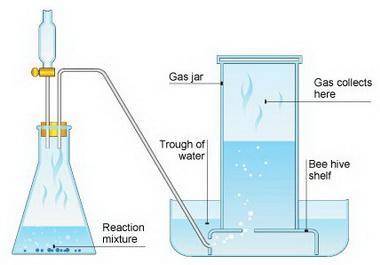
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 743 torr. if the hydrogen gas is produced by the reaction of aluminum with hydrochloric acid:
2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g)
what volume of hydrogen gas will be collected if 1.35 g al(s) reacts with excess hcl(aq)? express
your answer in liters.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 06:40, Science2019
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
Do you know the correct answer?
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 74...
Questions in other subjects:

Geography, 20.11.2020 04:30

English, 20.11.2020 04:30




Mathematics, 20.11.2020 04:30