
Chemistry, 20.11.2020 04:30, jalenclarke25
Please help! The data table below shows the rate of the following overall reaction based on different initial concentration values: 4X + 3Y + 2Z --> products. Determine the rate law equation and the rate constant with proper units.
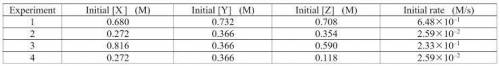

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
Do you know the correct answer?
Please help!
The data table below shows the rate of the following overall reaction based on differe...
Questions in other subjects:


Biology, 20.03.2020 06:38


Mathematics, 20.03.2020 06:38


History, 20.03.2020 06:38



History, 20.03.2020 06:39






