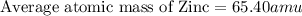2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu; 27.90% of 66zn with an atomic weight of 65.926 amu; 4.10% of 67zn with an atomic weight of 66.927 amu; 18.75% of 68zn with an atomic weight of 67.925 amu; and 0.62% of 70zn with an atomic weight of 69.925 amu. calculate the average atomic weight of zn.

Answers: 1
Similar questions

Physics, 11.07.2019 19:30, dbegay36
Answers: 1

Chemistry, 10.09.2019 19:30, dantedelafuente
Answers: 3

Chemistry, 13.09.2019 03:10, nikkera03
Answers: 1
Do you know the correct answer?
2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu...
Questions in other subjects:

Mathematics, 09.04.2021 22:10

Mathematics, 09.04.2021 22:10

Mathematics, 09.04.2021 22:10

Mathematics, 09.04.2021 22:10

Chemistry, 09.04.2021 22:10

Mathematics, 09.04.2021 22:10

Biology, 09.04.2021 22:10

Mathematics, 09.04.2021 22:10


Mathematics, 09.04.2021 22:10


 .....(1)
.....(1)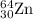 isotope:
isotope: isotope:
isotope: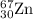 isotope:
isotope: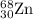 isotope:
isotope: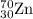 isotope:
isotope:![\text{Average atomic mass of Zinc}=[(63.929\times 0.4863)+(65.926\times 0.2790)+(66.927\times 0.0410)+(67.925\times 0.1875)+(69.925\times 0.0062)]](/tpl/images/0237/0119/27e73.png)