

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
2.4 bromium has two naturally occurring isotopes: 79br, with an atomic weight of 78.918 amu, and 81...
Questions in other subjects:



English, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31


History, 11.12.2019 15:31

Biology, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31



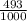 81Br and
81Br and 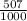 79BR
79BR




