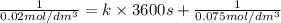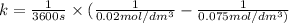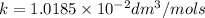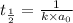Chemistry, 13.09.2019 21:20, haleybug100300
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 mol dm-3 in a and .03 mol dm-3 in b. after 1 hour, the concentration of a had fallen to .02 mol dm-3. a. calculate the rate constant. b. what is the half life of the reactant? answers: a. 16.2 dm3mol-hr-, 4.5e-3 dm3mol-s- b. 5.1e3s, 2.1e3 s

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Do you know the correct answer?
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 m...
Questions in other subjects:


English, 11.01.2021 21:00




English, 11.01.2021 21:00

Physics, 11.01.2021 21:00


Health, 11.01.2021 21:00

History, 11.01.2021 21:00


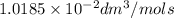 .
. is the half life of the reactant.
is the half life of the reactant.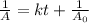
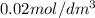
 = Initial concentration =
= Initial concentration =