Chemistry, 06.09.2019 22:30, muanghoih14
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements is true? a) the reaction is spontaneous above 276 k. b) the reaction is spontaneous below 276 k. c) the reaction will never reach equilibrium. d) the reaction will never be spontaneous.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2
Do you know the correct answer?
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements...
Questions in other subjects:

Mathematics, 16.04.2020 03:03

Computers and Technology, 16.04.2020 03:03





Chemistry, 16.04.2020 03:03




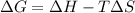
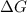 = Gibbs free energy
= Gibbs free energy 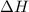 = enthalpy change = +29.3 kJ/mol =29300 J/mol
= enthalpy change = +29.3 kJ/mol =29300 J/mol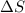 = entropy change = +106 J/molK
= entropy change = +106 J/molK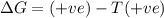
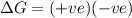
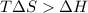 for reaction to be spontaneous
for reaction to be spontaneous