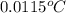Chemistry, 03.09.2019 02:30, 12martinkat
How much heat is produced when 100ml of 0.25 m hcl (density 1.00g/ml) and 200 ml of 0.150 m naoh (densty 1.00g/ml) are mixed?
hcl + naoh ? nacl + h2o ho298= -58kj
if both solutions are the same temperatureand heat capaciy of the products is 4.19 j/gc, how much will the temperature increase? what assumption did you make in your calculation?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, qwerty8364
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
Do you know the correct answer?
How much heat is produced when 100ml of 0.25 m hcl (density 1.00g/ml) and 200 ml of 0.150 m naoh (de...
Questions in other subjects:











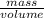
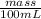
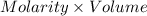
 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)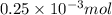
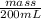
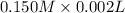 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)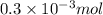
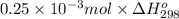
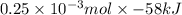
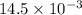 kJ
kJ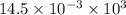 J
J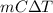
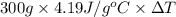
 =
=