

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
Do you know the correct answer?
Magnesium will burn in air to form both mg3n2 and mgo. what mass of each product would be found if b...
Questions in other subjects:



Computers and Technology, 12.02.2020 02:05



Advanced Placement (AP), 12.02.2020 02:05





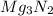 formed is 1.587 grams.
formed is 1.587 grams.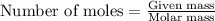 .....(1)
.....(1)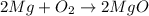
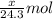
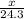 moles of magnesium will produce
moles of magnesium will produce 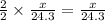 moles of MgO
moles of MgO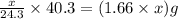
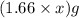 = X grams
= X grams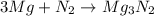
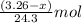
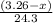 moles of magnesium will produce
moles of magnesium will produce 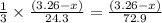 moles of
moles of ![Mg_3N_2=\frac{(3.26-x)}{72.9}\times 101=[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/e57b9.png)
![[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/79581.png) = Y grams
= Y grams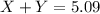
![(1.66\times x)+[(3.26-x)\times 1.38]=5.09\\\\x=2.11g](/tpl/images/0221/4247/08016.png)
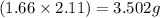
![Mg_3N_2=[(3.26-2.11)\times 1.38]=1.587g](/tpl/images/0221/4247/42984.png)




