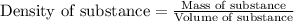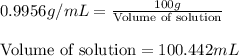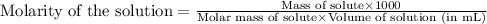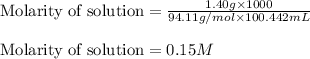Chemistry, 02.09.2019 19:30, thicklooney
Athroat spray is 1.40% by mass phenol, c6h5oh in water. if the solution has a density of 0.9956 g/ml, calculate the molarity of the solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
Do you know the correct answer?
Athroat spray is 1.40% by mass phenol, c6h5oh in water. if the solution has a density of 0.9956 g/ml...
Questions in other subjects:

Physics, 06.11.2020 22:00

Spanish, 06.11.2020 22:00

Social Studies, 06.11.2020 22:00


Health, 06.11.2020 22:00


Mathematics, 06.11.2020 22:00

Health, 06.11.2020 22:00

Mathematics, 06.11.2020 22:00

Physics, 06.11.2020 22:00