
Chemistry, 02.09.2019 17:30, wesillyskeletons
The equilibrium reaction below has the kc = 0.254 at 25°c. if the temperature of the system at equilibrium is decreased to 0°c, how and for what reason will the equilibrium shift? also show and explain how and why the kc value will change.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
Do you know the correct answer?
The equilibrium reaction below has the kc = 0.254 at 25°c. if the temperature of the system at equil...
Questions in other subjects:

Medicine, 30.08.2019 00:30



Chemistry, 30.08.2019 00:30


Mathematics, 30.08.2019 00:30

Business, 30.08.2019 00:30




![Kc=\frac{[products]}{[reagents]}](/tpl/images/0221/0240/214f4.png) rised to the power of their number of moles in the balanced reaction. When you have a system at equilibrium with Kc < 1, it means [products] < [reagents] and the system needs energy to react, so if you decrease tempeture the equilibrium shifts towards reagents and less products will be created.
rised to the power of their number of moles in the balanced reaction. When you have a system at equilibrium with Kc < 1, it means [products] < [reagents] and the system needs energy to react, so if you decrease tempeture the equilibrium shifts towards reagents and less products will be created.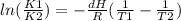 where we can see that if we decrease temperature (this is T2<T1) in consecuense K2<K1 and reaction doesn´t happen.
where we can see that if we decrease temperature (this is T2<T1) in consecuense K2<K1 and reaction doesn´t happen.




