
Chemistry, 30.08.2019 00:30, abdominguez7307
What is added to balance hydrogen and oxygen atoms in an oxidation reduction reaction in acidic solution?

Answers: 1
Similar questions

Chemistry, 31.07.2019 10:30, UsedForSchool2018
Answers: 2

Chemistry, 08.08.2019 04:20, JulietteRosso995
Answers: 3


Chemistry, 06.10.2019 12:00, cmob13
Answers: 1
Do you know the correct answer?
What is added to balance hydrogen and oxygen atoms in an oxidation reduction reaction in acidic solu...
Questions in other subjects:

Mathematics, 26.03.2021 05:50

Mathematics, 26.03.2021 05:50


Mathematics, 26.03.2021 05:50





Mathematics, 26.03.2021 05:50

Biology, 26.03.2021 05:50


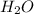 ) where there are less number of oxygen atoms. The number of the water molecules added should be equal to the number of the oxygen atoms short on either side of the reaction.
) where there are less number of oxygen atoms. The number of the water molecules added should be equal to the number of the oxygen atoms short on either side of the reaction.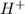 ) where there are less number of hydrogen atoms. The number of the Protons added should be equal to the number of the hydrogen atoms short on either side of the reaction.
) where there are less number of hydrogen atoms. The number of the Protons added should be equal to the number of the hydrogen atoms short on either side of the reaction.



