

Answers: 1
Similar questions


Chemistry, 10.09.2019 00:30, bluetigerbird5323
Answers: 3


Chemistry, 20.10.2019 00:30, ranamontana98
Answers: 1
Do you know the correct answer?
Given the following balanced equation, determine the rate of reaction with respect to [cl2]. if the...
Questions in other subjects:











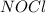 is,
is, 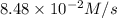
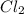 =
= 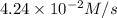
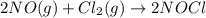
![\text{Rate of disappearance}=-\frac{1}{2}\frac{d[NO]}{dt}=-\frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/aee73.png)
![\text{Rate of formation}=\frac{1}{2}\frac{d[NOCl]}{dt}](/tpl/images/0155/2847/25db6.png)
![\frac{1}{2}\frac{d[NOCl]}{dt}=-\frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/f6a9b.png)
![\frac{d[NOCl]}{dt}=2\times \frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/71d98.png)
![\frac{d[NOCl]}{dt}=2\times (4.24\times 10^{-2}M/s)=8.48\times 10^{-2}M/s](/tpl/images/0155/2847/fdae3.png)




