
Chemistry, 10.09.2019 00:30, bluetigerbird5323
Rate law equation the rate of a chemical reaction depends on the concentrations of the reactants. for the general reaction between a and b, aa+bb⇌cc+dd the dependence of the reaction rate on the concentration of each reactant is given by the equation called the rate law: rate=k[a]m[b]n where k is a proportionality constant called the rate constant. the exponent m determines the reaction order with respect to a, and n determines the reaction order with respect to b. the overall reaction order equals the sum of the exponents (m+n). what is the reaction order with respect to a?

Answers: 3
Similar questions

Chemistry, 10.09.2019 18:20, aekent2003
Answers: 2
Do you know the correct answer?
Rate law equation the rate of a chemical reaction depends on the concentrations of the reactants. fo...
Questions in other subjects:

English, 03.03.2021 19:30

Mathematics, 03.03.2021 19:30


Social Studies, 03.03.2021 19:30



Mathematics, 03.03.2021 19:30

Mathematics, 03.03.2021 19:30


Mathematics, 03.03.2021 19:30


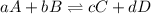
![Rate=k[A]^m[B]^n](/tpl/images/0226/2472/b2185.png)




