

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2


Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
Do you know the correct answer?
At 85°c, the vapor pressure of a is 566 torr and that of b is 250 torr. calculate the composition of...
Questions in other subjects:



Chemistry, 20.09.2019 10:50


Chemistry, 20.09.2019 10:50



Mathematics, 20.09.2019 10:50


Mathematics, 20.09.2019 10:50


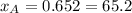 %
%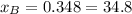 %
%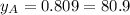 %
%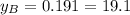 %
%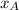 and
and 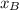 molar fractions can be calculated as:
molar fractions can be calculated as: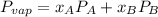
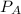 and
and 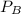 are the vapor pressures of the pure compounds. A substance boils when its vapor pressure is equal to the pressure under it is; so it boils when
are the vapor pressures of the pure compounds. A substance boils when its vapor pressure is equal to the pressure under it is; so it boils when  . When the pressure is 0.60 atm, the vapor pressure has to be the same if the mixture is boiling, so:
. When the pressure is 0.60 atm, the vapor pressure has to be the same if the mixture is boiling, so: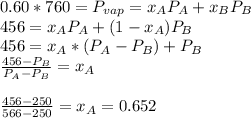
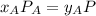 , where P is the total pressure and y is the fraction in the vapor phase, so:
, where P is the total pressure and y is the fraction in the vapor phase, so: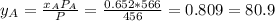 %
%



