
Chemistry, 18.07.2019 23:30, kalialee2424
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer solution with ph 9.50. the final concentration of na2co3 in this solution is 0.10 m. pka1 = 6.37 and pka2 = 10.33 for h2co3.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 23.06.2019 10:00, JuanTorres7
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
Do you know the correct answer?
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer sol...
Questions in other subjects:


Social Studies, 06.02.2022 04:10

Mathematics, 06.02.2022 04:10

Mathematics, 06.02.2022 04:10

Social Studies, 06.02.2022 04:10

Mathematics, 06.02.2022 04:10


English, 06.02.2022 04:10

Mathematics, 06.02.2022 04:10

Social Studies, 06.02.2022 04:10


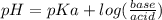
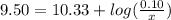
 is the concentration of sodium bicarbonate)
is the concentration of sodium bicarbonate)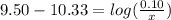
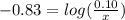
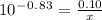
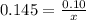
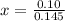
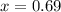
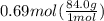
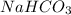 will be required.
will be required. 



