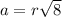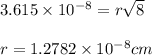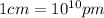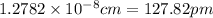Chemistry, 05.07.2019 20:20, bryson9604
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) calculate the mass of one unit cell of copper (in grams) b.) calculate the volume of the copper unit cell (in cm3). c.) calculate the edge length of the unit cell (in cm). d.) calculate the radius of a copper atom (in pm).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Do you know the correct answer?
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) ca...
a.) ca...
Questions in other subjects:



Mathematics, 05.03.2021 18:00

Mathematics, 05.03.2021 18:00






Mathematics, 05.03.2021 18:00


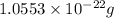
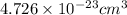
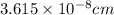
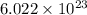 number of atoms.
number of atoms.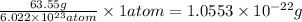
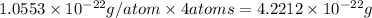
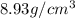
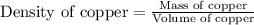
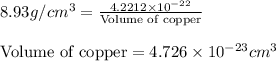
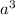
![\sqrt[3]{4.726\times 10^{-23}}cm^3=3.615\times 10^{-8}cm](/tpl/images/0055/3009/53021.png)