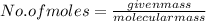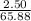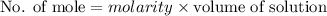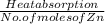Chemistry, 29.06.2019 00:20, webbjalia04
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic acid in a calorimeter. the total heat capacity of the calorimeter and solution is 448 j/k. the observed increase in temperature is 21.1 k at a constant pressure of one bar. calculate the standard enthalpy of reaction using these data. zn(s)+2hbr(aq)⟶znbr2(aq)+h2(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Do you know the correct answer?
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic aci...
Questions in other subjects:


History, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01

English, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01