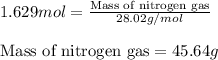Chemistry, 27.06.2019 19:30, 38saferguson
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n204 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 92.02 g/mol, n2h4 32.05 g/mol n204) 2 n2h4(1)3 n2(g) + 4 h2o(g)

Answers: 2
Similar questions

Chemistry, 19.07.2019 17:20, Raekwon3232
Answers: 1

Chemistry, 22.07.2019 02:01, salam6809
Answers: 1
Do you know the correct answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions in other subjects:

Mathematics, 06.05.2021 06:20

English, 06.05.2021 06:20


Biology, 06.05.2021 06:20

Mathematics, 06.05.2021 06:20



Mathematics, 06.05.2021 06:20

Mathematics, 06.05.2021 06:20


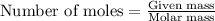 .....(1)
.....(1)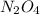
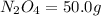
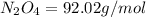
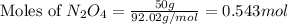
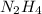
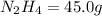
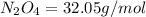
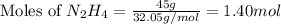
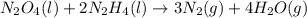
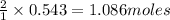 of
of 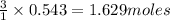 of nitrogen gas.
of nitrogen gas.