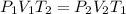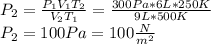Chemistry, 30.06.2019 07:20, redrhino27501
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square meter. if the temperature was reduced to 250 kelvins, and the volume raised to 9 liters, what was the resulting pressure ? (a) 100 newtons/m^2 (b) 150 newtons/m^2 (c) 450 newtons/m^2 (d) 900 newtons/m^2 (e) none of these

Answers: 2
Similar questions

Physics, 21.06.2019 23:30, izzyisawesome5232
Answers: 3

Chemistry, 25.08.2019 17:20, novesparks
Answers: 2

Physics, 21.09.2019 07:50, babbybronx
Answers: 1

Chemistry, 11.10.2019 04:00, kylie252
Answers: 3
Do you know the correct answer?
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square m...
Questions in other subjects:

Mathematics, 25.10.2019 04:43



Mathematics, 25.10.2019 04:43

Biology, 25.10.2019 04:43

Mathematics, 25.10.2019 04:43




Mathematics, 25.10.2019 04:43