Chemistry, 25.08.2019 17:20, novesparks
1. when the kelvin temperature of an enclosed gas doubles, the particles of the gas (1 point)
move faster
strike the walls of the container with less force
decrease in average kinetic energy
decrease in volume
2. the volume of a gas is reduced from 4 l to 0.5 l while the temperature is held constant. how (1 point)
2. the volume of a gas is reduced from 4 l to 0.5 l while the temperature is held constant. how
does the gas pressure change?
(1 point)
it increases by a factor of four.
it decreases by a factor of eight.
it increases by a factor of eight.
it increases by a factor of two.
3. charles's law states that (1 point)
the pressure of a gas is inversely proportional to its temperature in kelvins
the volume of a gas is directly proportional to its temperature in kelvins
the pressure of a gas is directly proportional to its temperature in kelvins
the volume of a gas is inversely proportional to its temperature in kelvins
4. a sample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c? (1 point)
10.6 ml
27 ml
36 ml
8.0 ml
5. the combined gas law relates which of the following? (1 point)
pressure and volume only
temperature and pressure only
volume and temperature only
temperature, pressure, and volume

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
Do you know the correct answer?
1. when the kelvin temperature of an enclosed gas doubles, the particles of the gas (1 point)
...
...
Questions in other subjects:

History, 11.10.2019 13:20

Mathematics, 11.10.2019 13:20

English, 11.10.2019 13:20






History, 11.10.2019 13:20


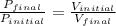
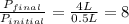
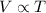
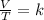
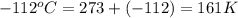
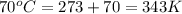
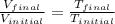
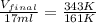
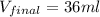
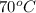 is, 36 ml
is, 36 ml