Which statement describes the potential energy diagram of an endothermic reaction?
a. the potential energy of the products is equal to the potential energy of the reactants.
b. the potential energy of the reactants is greater than the potential energy of the products.
c. the activation energy of the reactants is less than the activation energy of the products.
d. the potential energy of the products is greater than the potential energy of the reactants.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, demetriascott20
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
Do you know the correct answer?
Which statement describes the potential energy diagram of an endothermic reaction?
a. the po...
a. the po...
Questions in other subjects:

History, 10.05.2021 06:50


English, 10.05.2021 06:50

Chemistry, 10.05.2021 06:50

Health, 10.05.2021 06:50


English, 10.05.2021 06:50




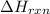 comes out to be positive.
comes out to be positive.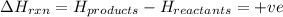
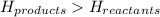 for endothermic reactions
for endothermic reactions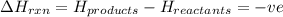
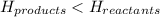 for exothermic reactions
for exothermic reactions