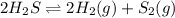Chemistry, 27.06.2019 00:30, cobyontiveros
How does the equilibrium change with the removal of hydrogen (h2) gas from this equation? 2h2s ⇌ 2h2(g) + s2(g) a. equilibrium shifts right to produce more product. b. equilibrium shifts left to produce more reactant. c. equilibrium shifts right to produce less product. d. equilibrium shifts left to produce less reactant.

Answers: 2
Similar questions

Chemistry, 02.07.2019 05:00, missymiss4273
Answers: 1

Chemistry, 03.07.2019 01:00, kelseatuttleni
Answers: 1

Chemistry, 09.10.2019 00:30, mika08
Answers: 2
Do you know the correct answer?
How does the equilibrium change with the removal of hydrogen (h2) gas from this equation? 2h2s ⇌ 2h...
Questions in other subjects:

Physics, 09.01.2021 04:40

Mathematics, 09.01.2021 04:40


Biology, 09.01.2021 04:40

Mathematics, 09.01.2021 04:40

Mathematics, 09.01.2021 04:40

Mathematics, 09.01.2021 04:40


Mathematics, 09.01.2021 04:40

Mathematics, 09.01.2021 04:40