
Chemistry, 29.06.2019 08:00, jameslinimk
For an exothermic reaction at equilibrium, how will increasing the temperature affect keq? a. keq will increase. b. keq will decrease. c. keq will stay the same. d. keq will double.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Do you know the correct answer?
For an exothermic reaction at equilibrium, how will increasing the temperature affect keq? a. keq w...
Questions in other subjects:











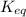 will decrease.
will decrease.![K_{eq} = \frac{[C]^{c}\cdot [D]^d}{[A]^{a} \cdot [B]^{b}}](/tpl/images/0030/3102/48db3.png) , where [A], [B], [C], and [D] are equilibrium concentrations of the four species. Converting some products back to reactants increases [A] and [B] while reducing [C] and [D]. As a result,
, where [A], [B], [C], and [D] are equilibrium concentrations of the four species. Converting some products back to reactants increases [A] and [B] while reducing [C] and [D]. As a result, 



