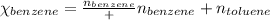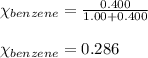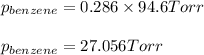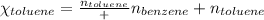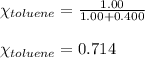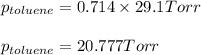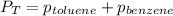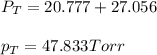Chemistry, 21.02.2020 17:21, labrandonanderson00
Benzene, C6H6, and toluene, C6H5CH3, form an ideal solution. The vapor pressure of benzene is 94.6 Torr and that of Topic 5C Exercises 372 Topic 5C Phase Equilibria in Two-Component Systems toluene is 29.1 Torr at 25 8C. What is the vapor pressure of each component at 25 8C and what is the total vapor pressure of a mix- ture of 1.00 mol benzene and 0.400 mol toluene at 25 8C?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
Do you know the correct answer?
Benzene, C6H6, and toluene, C6H5CH3, form an ideal solution. The vapor pressure of benzene is 94.6 T...
Questions in other subjects:




Physics, 05.02.2021 01:10






Mathematics, 05.02.2021 01:10


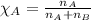
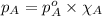 ........(1)
........(1)