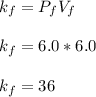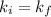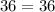Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0 atm, the volume of the gas decreases to 6.0l. find the two constants ki, the initial value of k, and kf, the final value of k, to verify if whether the gas obeys boyle’s law.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
Do you know the correct answer?
Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0...
Questions in other subjects:


History, 01.12.2020 19:00




History, 01.12.2020 19:00


English, 01.12.2020 19:00


Biology, 01.12.2020 19:00


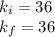
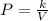
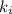 :
: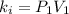
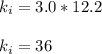
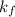 :
: