
Chemistry, 03.07.2019 17:30, enitramedouard12
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane needed to produce 90.9 g of carbon dioxide. calculate the mass of butane needed to produce 90.9 g of carbon dioxide.

Answers: 1
Similar questions

Physics, 01.07.2019 15:00, ryleerose255
Answers: 1


Chemistry, 21.08.2019 18:40, serenityarts123
Answers: 1

Chemistry, 15.10.2019 18:40, dylankrenek
Answers: 3
Do you know the correct answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane ne...
Questions in other subjects:


Mathematics, 10.02.2021 20:50

Mathematics, 10.02.2021 20:50


History, 10.02.2021 20:50






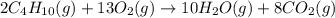
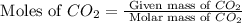
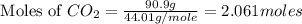
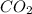 produced from 2 moles
produced from 2 moles 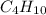
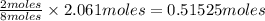 of
of 



