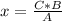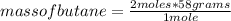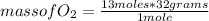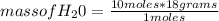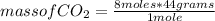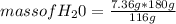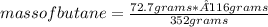Chemistry, 21.08.2019 18:40, serenityarts123
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane reacts with excess oxygen.. express your answer to three significant figures and include the appropriate units.. calculate the mass of butane needed to produce 72.7g of carbon dioxide.. express your answer to three significant figures and include the appropriate units.. how do you do it?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
Do you know the correct answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane react...
Questions in other subjects:

Mathematics, 22.07.2019 15:00

Physics, 22.07.2019 15:00

Mathematics, 22.07.2019 15:00





History, 22.07.2019 15:00


Mathematics, 22.07.2019 15:00