
Chemistry, 10.07.2019 00:30, chrisraptorofficial
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reactions stop at the appropriate concentrations. at equilibrium, the forward and reverse reactions continue indefinitely. at equilibrium the rate of reaction of products divided by the rate of reaction of reactants equal the equilibrium constant, k.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
Do you know the correct answer?
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reac...
Questions in other subjects:



Mathematics, 05.04.2021 05:50

Chemistry, 05.04.2021 05:50

Mathematics, 05.04.2021 05:50




Mathematics, 05.04.2021 05:50

Mathematics, 05.04.2021 05:50


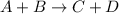
![k = \frac{[C][D]}{[A][B]}](/tpl/images/0071/3436/05b05.png)
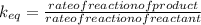
![\frac{\frac{d[C][D]}{dt}}{\frac{d[A][B]}{dt}}](/tpl/images/0071/3436/6ae5f.png)





