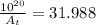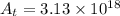Answers: 1
Similar questions

Physics, 06.09.2019 20:10, ashl3yisbored
Answers: 1

Chemistry, 20.09.2019 17:20, bellabasketball5412
Answers: 2

Chemistry, 09.10.2019 02:00, tylerineedhelp
Answers: 2

Chemistry, 03.11.2019 06:31, jcduarte
Answers: 2
Do you know the correct answer?
Afirst-order reaction has a half-life of 20.0 minutes. starting with 1.00 × 1020 molecules of reacta...
Questions in other subjects:


Computers and Technology, 16.10.2019 18:30


Social Studies, 16.10.2019 18:30


Computers and Technology, 16.10.2019 18:30



Mathematics, 16.10.2019 18:30


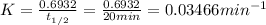
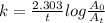
 , time is 100 min thus, putting the values to calculate number of reactant at time 100 min,
, time is 100 min thus, putting the values to calculate number of reactant at time 100 min,![0.03466 min^{-1}=\frac{2.303}{100 min}log\frac{[10^{20}]}{A_{t}}](/tpl/images/0071/4315/f70c9.png)