
Chemistry, 10.07.2019 08:30, keidyhernandezm
Calculate the molar concentration of the polycyclic aromatic hydrocarbon (pah), anthracene (178.23 g/mol), that was found in a well water sample at a concentration of 6.92 ppb. assume the density of the water is 1.00 g/ml.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
Do you know the correct answer?
Calculate the molar concentration of the polycyclic aromatic hydrocarbon (pah), anthracene (178.23 g...
Questions in other subjects:


Social Studies, 03.12.2020 14:20

History, 03.12.2020 14:20

Mathematics, 03.12.2020 14:20

Mathematics, 03.12.2020 14:20

Health, 03.12.2020 14:20



Biology, 03.12.2020 14:20


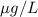 .
.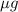 in 1 liter of sample.
in 1 liter of sample.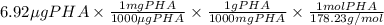
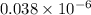 mol PHA (Anthracene)
mol PHA (Anthracene)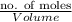
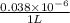
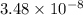 M
M



