
Chemistry, 03.12.2020 14:20, babyduckies37
25.0cm3 of s saturated potassium hydroxide is neutralized by 35.0cm3 of hydrogen chloride acid of concentration 0.75 mol/dm3. Calculate the concentration of potassium hydroxide solution. Please help, will give brainliest, unhelpful answers will get reported.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Do you know the correct answer?
25.0cm3 of s saturated potassium hydroxide is neutralized by 35.0cm3 of hydrogen chloride acid of co...
Questions in other subjects:










Mathematics, 19.11.2019 19:31


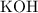 solution: approximately
solution: approximately 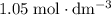 .
. 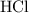 solution is in the unit
solution is in the unit 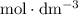 . However, the unit of the two volumes is
. However, the unit of the two volumes is 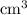 . Convert the unit of the two volumes to
. Convert the unit of the two volumes to 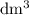 to match the unit of concentration.
to match the unit of concentration.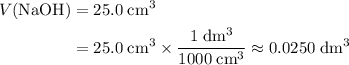 .
.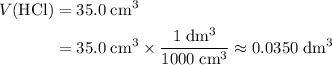 .
.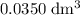 of
of 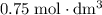
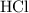 solution:
solution: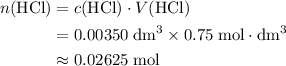 .
.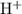 .
.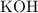 formula unit would react with up to one
formula unit would react with up to one 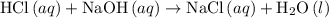 .
.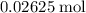 of
of 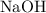 formula units. That is:
formula units. That is: 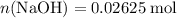 .
.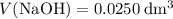 and
and 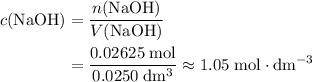 .
.



