
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate of barium sulfate (fw: 233.4 g/mol). the dry solid weighs 0.397 g. use this mass and the dilution volumes to calculate the actual molarity of the sulfuric acid in the initial solution. (previous question ask: 10.00 ml of approximately 6 m sulfuric acid is transferred to a 100ml volumetric flask and diluted to the mark with distilled water and mixed. then 10.00 ml of this solution was further diluted to 100 ml. the molarity of the final solution was 0.06 m).

Answers: 1
Similar questions

Chemistry, 16.09.2019 13:10, AllenTTG
Answers: 1


Chemistry, 27.10.2019 04:43, amberskids2
Answers: 1

Chemistry, 20.11.2019 21:31, smithsa10630
Answers: 3
Do you know the correct answer?
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate...
Questions in other subjects:

History, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

History, 04.02.2021 20:10

Biology, 04.02.2021 20:10


English, 04.02.2021 20:10



Mathematics, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10


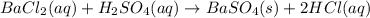
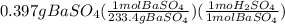
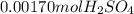
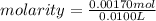
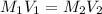
![Y=\frac{0.170M*100mL}{10.0mL}Y = 1.70MLet's do the similar calculations to find out the actual molarity of the original acid solution. Let's say the molarity of the original acid solution is X. 10.0 mL of it were taken and diluted to 100 mL on adding water. The molarity is 1.70M as is calculated in the above step. Let's plug in the values in the molarity equation again to solve it for X as:X(10.0mL) = 1.70M(100mL)[tex]X=\frac{1.70M*100mL}{10.0mL}](/tpl/images/0073/9434/d3677.png)




