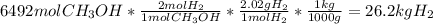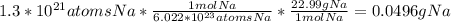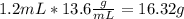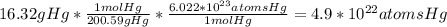Chemistry, 14.07.2019 04:30, thegamingkid914
Asap (: need with these 3 1. what mass of h2 would be needed to produce 208kg of methanol? 2. how many grams do 1.3 x 10^21 atoms of sodium weigh? 3. using the average atomic mass, calculate the number of atoms present in 1.2 ml of liquid mercury. (density= 13.6g/ml)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, duracohack
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1


Do you know the correct answer?
Asap (: need with these 3 1. what mass of h2 would be needed to produce 208kg of methanol? 2. h...
Questions in other subjects:


Mathematics, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01

Health, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01






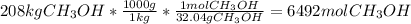
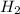 that would produce 208 kg methanol =
that would produce 208 kg methanol =