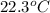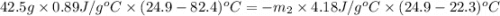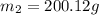Physics, 29.07.2019 02:00, christabell0303
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/goc) initially at 22.3oc. the final temperature of the water is 24.9oc. calculate the mass of water in the calorimeter. ignore significant figures for this problem.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, jonquil201
The us government wants to allocate billions of dollars in the next 10 years to assure our future energy security. the funds will be spread among a variety of possible energy resources. where do you think the government should put the greatest support: solar energy, wind energy, clean coal, oil exploration, gas exploration, or a combination of sources? are there other efforts that should be explored? support your position with cited information for both questions.
Answers: 2

Physics, 22.06.2019 10:00, starsinopoli13
How are the crust and the inner core alike? a) they are both solid. b) they both have the same temperature. c) they are both under the same pressure. d) they are both very close to the center of the earth.
Answers: 1


Physics, 22.06.2019 12:50, shollydot1379
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3
Do you know the correct answer?
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and...
Questions in other subjects:



Chemistry, 12.04.2021 18:40




Geography, 12.04.2021 18:40

History, 12.04.2021 18:40


Geography, 12.04.2021 18:40


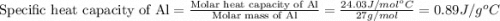
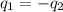
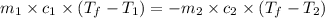
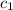 = specific heat of aluminum =
= specific heat of aluminum = 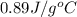
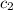 = specific heat of water =
= specific heat of water = 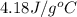
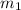 = mass of Al = 42.5 g
= mass of Al = 42.5 g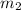 = mass of water = ?
= mass of water = ?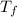 = final temperature of water =
= final temperature of water = 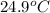
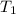 = initial temperature of Al =
= initial temperature of Al = 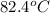
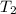 = initial temperature of water =
= initial temperature of water =