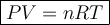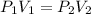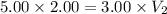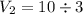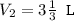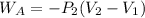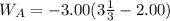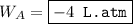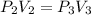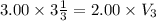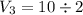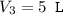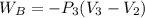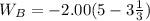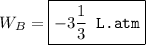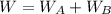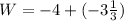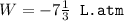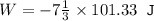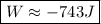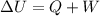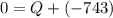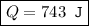Physics, 15.07.2019 19:50, oliviaschmitt0
An ideal gas is allowed to expand isothermally from 2.00 l at 5.00 atm in two steps: a. against a constant external pressure of 3.00 atm, followed by b. against a constant external pressure of 2.00 atm. calculate q and w. (101.33 j = 1 l atm)

Answers: 1
Similar questions

Physics, 03.07.2019 02:30, janai3602
Answers: 3


Physics, 18.07.2019 02:20, xxaurorabluexx
Answers: 1

Chemistry, 09.10.2019 20:00, natannale
Answers: 2
Do you know the correct answer?
An ideal gas is allowed to expand isothermally from 2.00 l at 5.00 atm in two steps: a. against a c...
Questions in other subjects:


Mathematics, 14.02.2021 21:20

Mathematics, 14.02.2021 21:20

English, 14.02.2021 21:20

Mathematics, 14.02.2021 21:20


Medicine, 14.02.2021 21:20