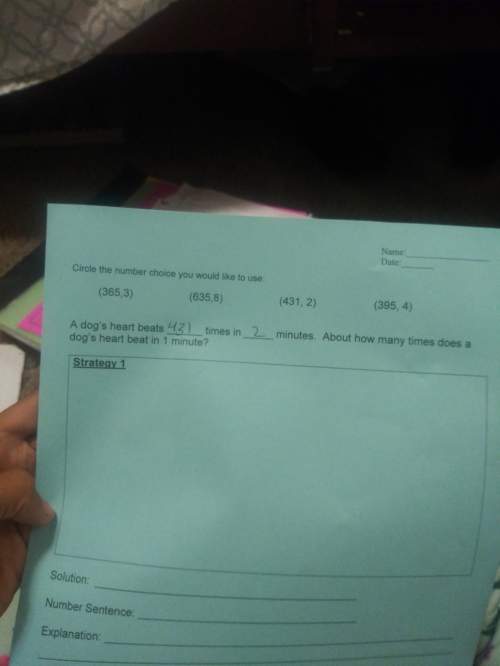
A gas undergoes isochoric heating, during which it gains 5470 J of heat and attains a pressure of 3.45 X 105 Pa. Following this, it experiences an isobaric compression that is also adiabatic, in which its volume decreases by 6.84 X 10-3 m3. Find the total change in the internal energy of the gas for this two-step process. Be sure to include the algebraic sign ( or -) of the total change in the internal energy.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 23:00, POOPINgligma
Atrain departs from its station at a constant acceleration of 5 m/s. what is the speed of the train at the end of 20s?
Answers: 1

Physics, 23.06.2019 02:00, nativebabydoll35
Frequency of electromagnetic waves that a radio station is assigned. what do you call this type of wave?
Answers: 2

Physics, 23.06.2019 02:40, ayoismeisalex
When doing scientific research ,the sources used should be ?
Answers: 1

Physics, 23.06.2019 13:00, sayedabdullah
Aball is fired with an initial velocity of 500 m/s at an angle of 30 degrees above the horizontal across a level field. a. what are the horizontal and vertical components of the initial velocity. b. calculate the maximum height, the time of flight, and the range of the ball.
Answers: 1
Do you know the correct answer?
A gas undergoes isochoric heating, during which it gains 5470 J of heat and attains a pressure of 3....
Questions in other subjects:

Mathematics, 29.09.2020 22:01


Biology, 29.09.2020 22:01

History, 29.09.2020 22:01



History, 29.09.2020 22:01

Business, 29.09.2020 22:01