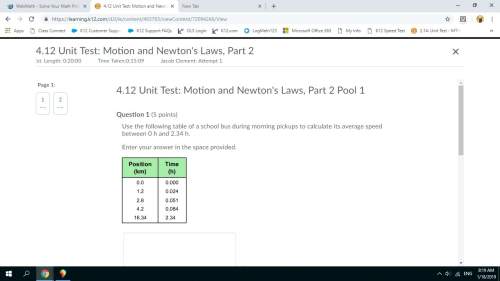
Physics, 08.09.2021 20:40, noobieplayerxd
A canister filled with 3.5 mol of single-atom helium gas has a temperature of
300 K. What is the approximate total internal energy of the gas? (Recall that
3
the equation for kinetic energy due to translation in a gas is: nRT; the
2
equation for kinetic energy due to rotation of a molecule in a gas is: nRT; and
R = 8.31 J/(mol-K).)

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 02:00, browneyedbaby20
The image shows a pendulum in simple harmonic motion the pendulum starts at a and swing to e
Answers: 3

Physics, 22.06.2019 05:00, unknowntay04
He viscosities of several liquids are being compared. all the liquids are poured down a slope with equal path lengths. the liquid with the highest viscosity will
Answers: 3

Physics, 22.06.2019 06:20, lolweapon
Atwo-stage air compressor operates at steady state, compression 10m^3/min of air from 100 kpa and 300k to 1200 kpa. an intercooler between the two stages cools the air to 300k at a constant pressure of 350 kpa. the compression processes are isentropic. a) calculate the power required to run the compressor, in kw b) compare the result to the power required for isentropic compression from the same inlet state to the same final pressure.
Answers: 1

Physics, 22.06.2019 14:00, mariahgriego4126
Why is rain likely when warm, moisture-laden air meets cold air? a) the lighter warm air will rise and cool down, causing condensation and rain. b) the cold air moves faster and pushes the warm air away, causing condensation and rain. c) the moisture in the warm air condenses on contact with the cold air, causing rain to fall. d) the cold air mixes with the warm air, reducing its temperature causing moisture to condense.
Answers: 1
Do you know the correct answer?
A canister filled with 3.5 mol of single-atom helium gas has a temperature of
300 K. What is the a...
Questions in other subjects:





Social Studies, 29.10.2020 19:30

Mathematics, 29.10.2020 19:30

Biology, 29.10.2020 19:30

Mathematics, 29.10.2020 19:30

Spanish, 29.10.2020 19:30