
Physics, 08.09.2021 18:40, KayleighMorganhopkin
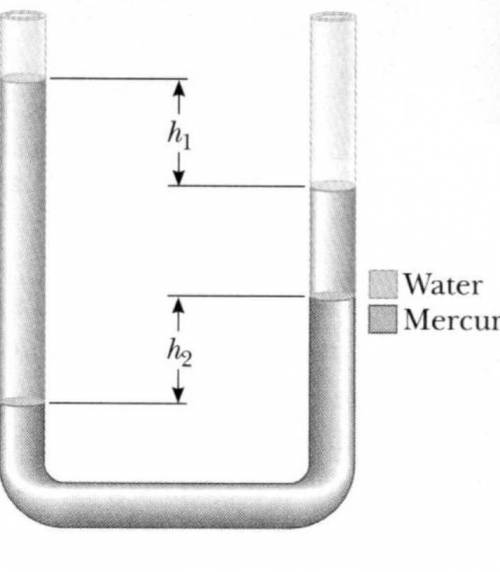
A U-tube uniform cross-sectional area and open to the atmosphere is partially filled with Mercury. Water then is then poured into both arms. If the equilibrium configuration of the tube is as shown in the figure with h2=1.04cm determine the value of h. There's no need to consider the weight of the of the extra air on the right-hand side

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 19:40, rileybaby34
Uranium has two naturally occurring isotopes. 238u has a natural abundance of 99.3% and 235u has an abundance of 0.7%. it is the rarer 235u that is needed for nuclear reactors. the isotopes are separated by forming uranium hexafluoride uf6, which is a gas, then allowing it to diffuse through a series of porous membranes. 235uf6 has a slightly larger rms speed than 238uf6 and diffuses slightly faster. many repetitions of this procedure gradually separate the two isotopes. what is the ratio of the rms speed of 235uf6 to that of 238uf6? express your answer to five significant figures.
Answers: 3

Physics, 23.06.2019 05:00, brianmondesir1owahud
Heating water for hot cocoa while sitting next to a campfire can be examples of conduction, convenction and radiation at the same time agree disagree it depends how you know
Answers: 2

Physics, 23.06.2019 07:30, Jasten
Neurons are components of the nervous system of the body that transmit signals as electrical impulses travel along their length. these impulses propagate when charge suddenly rushes into and then out of a part of the neutron called an axon. measurements have shown that, during the inflow part of this cycle approximately 5.6x10^11 na per meter, each with a charge of e enter the axon. how many coulombs of charge enter a 1.10 cm length of axon during this process
Answers: 2

Physics, 23.06.2019 09:30, triciajfive
According to rutherford's nuclear theory, most of the volume of an atom is , so the volume of a hydrogen atom cannot be mostly due to the proton. according to rutherford's nuclear theory, the core of an atom (nucleus) contains most of of an atom and is , so the majority of the mass of a fluorine atom cannot be due to its nine electrons. according to rutherford's nuclear theory, the number of negatively charged particles outside the nucleus the number of positively charged particles within the nucleus, so a nitrogen atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 electrons. word bank: dense, empty space, more than, mass, particles, the same as, less than, positively charged, negatively charged
Answers: 2
Do you know the correct answer?
A U-tube uniform cross-sectional area and open to the atmosphere is partially filled with Mercury. W...
Questions in other subjects:

Social Studies, 30.04.2021 22:20

Mathematics, 30.04.2021 22:20


Mathematics, 30.04.2021 22:20


Mathematics, 30.04.2021 22:20


History, 30.04.2021 22:20







