
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 10:00, andresduenas72
Aria drove to the store, did some shopping, and then came home. during maria's trip, when was her displacement equal to zero?
Answers: 1



Physics, 22.06.2019 22:30, dlsmith68
Asailboat is traveling to the right when a gust of wind causes the boat to accelerate leftward at 2.5m/s2 for 4s. after the wind stops, the sailboat is traveling to the left with a velocity of 3.0m/s. assuming the acceleration from the wind is constant, what was the initial velocity of the sailboat before the gust of wind? answer using a coordinate system where rightward is positive.
Answers: 1
Do you know the correct answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions in other subjects:

Biology, 02.08.2019 15:30

History, 02.08.2019 15:30

Geography, 02.08.2019 15:30

English, 02.08.2019 15:30

Geography, 02.08.2019 15:30


Geography, 02.08.2019 15:30

Biology, 02.08.2019 15:30



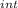 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T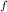 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 



